pvs
resources
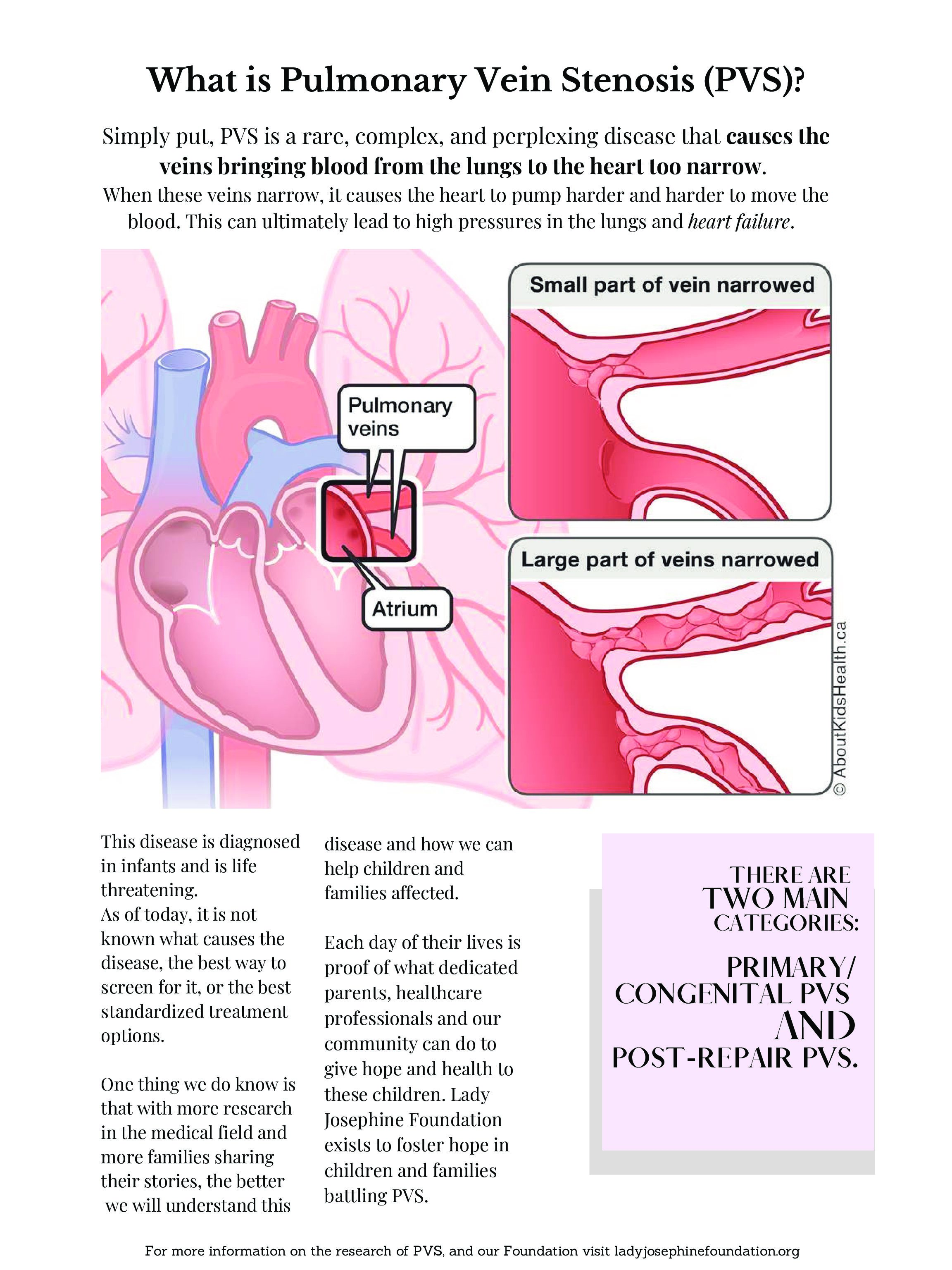
PVS is a serious disease resulting from a blockage in the blood vessels responsible for blood flow from the lungs back to the heart. It mimics a cancer-like growth within the veins that, even when eliminated with surgical intervention or medication, almost always makes an aggressive return. This disease cycle creates a chronic, life threatening condition that requires repeated, ongoing treatments and ultimately over time causes significant damage to the patient’s heart and lungs.
There are specialized centers across North America that have a specific interest in the research and treatment of PVS. The resources linked below are meant to provide information on PVS from reputable organizations. These resources are listed in no particular order as we have no affiliation or bias toward any of the organizations.
FOSTERING HOPE THROUGH KNOWLEDGE
The Lady Josephine Foundation was established to acknowledge our fight for answers that seemed unattainable, to celebrate our victories in a life that has defied the odds, and to support the many people that have and will continue to pursue a treatment plan to better the lives of children with PVS. We have created the Story Boards below to help raise awareness and foster knowledge on this progressive disease. Please take a moment and click on each image, and immerse yourself in a world where life is the goal. Get to know PVS and a few of our PVS families that with your contributions, you can directly help.
Donate Now
Make a fit to help make PVS kids better today and healthier tomorrow.